Beautiful Tips About Can Only Two Electrons That Spin In The Opposite Direction Exist
The Curious Case of the Spin Doctors (Electrons, That Is)
1. Spinning Around in the Quantum World
Ever wondered why matter doesn't just collapse in on itself? You can thank the quirky world of quantum mechanics, and more specifically, the electron's spin. It's not actually spinning like a tiny top, mind you. It's more like an intrinsic property, like having charge. This spin generates a tiny magnetic field, and that's where things get interesting. These subatomic particles behave in ways that often defy our everyday intuition, and electron spin is a prime example of this quantum weirdness.
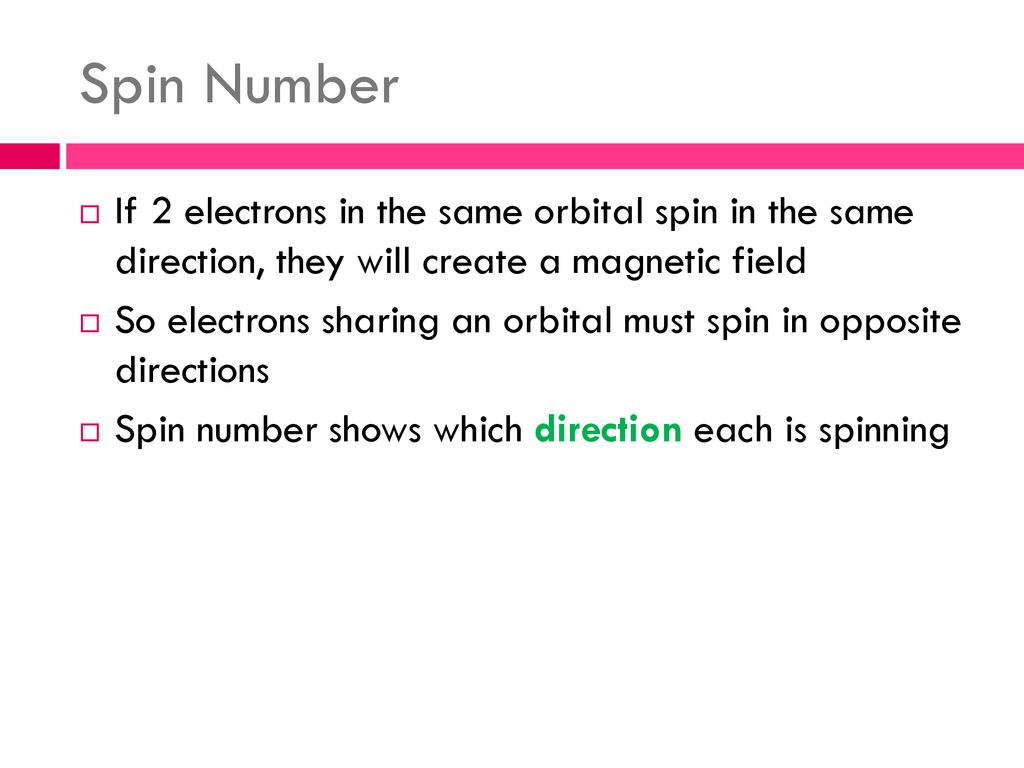
Imagine a crowded dance floor. To avoid bumping into each other, everyone needs to move in a coordinated way. Electrons are kind of like that, except they're following the rules of quantum mechanics. Electron spin is quantized, meaning it can only take on specific values. For each electron, there are only two allowed spin states: spin-up and spin-down. It's like flipping a coin 'heads' or 'tails', only on a subatomic scale.
So, what happens when electrons get together, like in an atom? Well, they tend to pair up. This pairing is governed by a fundamental principle known as the Pauli Exclusion Principle. This principle is so important it is a cornerstone of understanding the structure of matter itself.
This principle states that no two electrons in an atom can have the exact same set of quantum numbers. Quantum numbers are like an electron's unique address — its energy level, shape of its orbital, orientation in space, and, of course, its spin. So, if two electrons are sharing the same orbital (meaning they have the same energy level, shape, and orientation), they must have opposite spins. It's like a tiny quantum dance where only pairs with opposing moves are allowed.

Pauli Exclusion Principle
2. Keeping the Peace in Atomic Orbitals
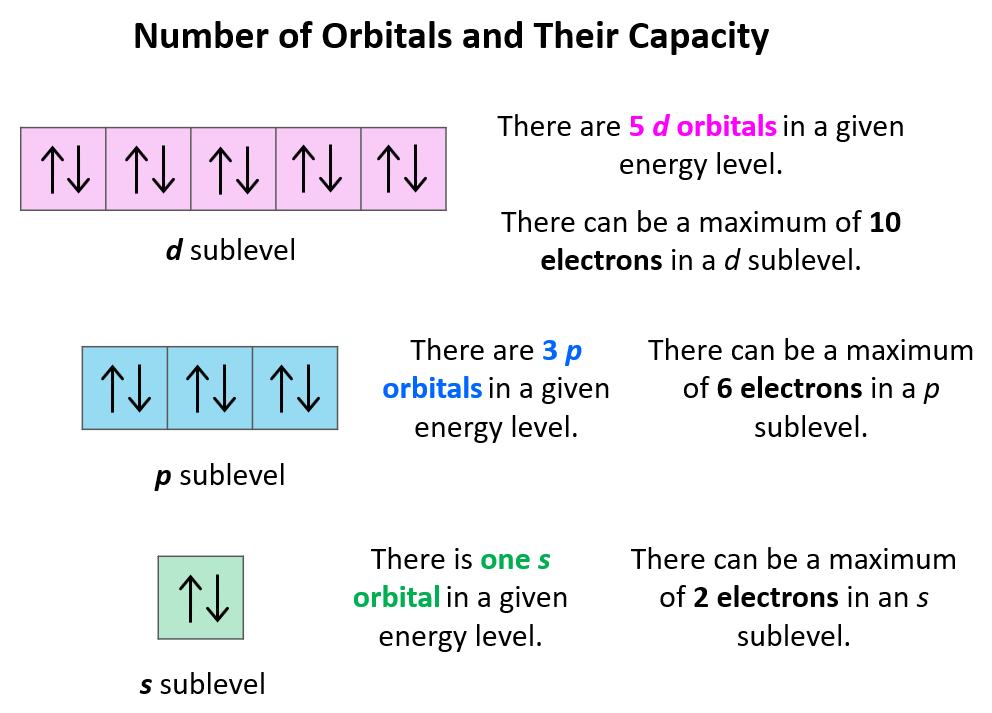
Think of the Pauli Exclusion Principle as a very strict bouncer at the "Electron Club." The bouncer only allows one electron per "seat" (quantum state). Now, in an atomic orbital, there are limited "seats." Each orbital can hold only a maximum of two electrons, but — and this is crucial — they must have opposite spins. It's the bouncer's rule to prevent overcrowding and maintain order.
Why this rule? Well, it comes down to the wave-like nature of electrons and their quantum behavior. If two electrons had the same spin and were in the same state, their wave functions would constructively interfere. This interference would lead to an unstable and energetically unfavorable situation. By having opposite spins, their wave functions interfere destructively, leading to a more stable configuration. It's like two out-of-sync radio waves canceling each other out.
The Pauli Exclusion Principle doesn't just apply to electrons. It applies to all particles called fermions, which are particles that have half-integer spin (1/2, 3/2, 5/2, etc.). Protons and neutrons, the particles that make up the atomic nucleus, are also fermions and follow this principle. Without it, matter as we know it would not exist. Atoms would collapse, and chemistry would be a completely different ballgame. No life, no universe — pretty important stuff, right?
So, when you're looking at an atom, remember that electrons aren't just randomly buzzing around. They're carefully arranged in orbitals, following the strict rules of the Pauli Exclusion Principle. It's a quantum ballet, elegantly choreographed to create the stable and diverse matter that makes up our world.
![[ANSWERED] 20 Two Electrons Are Forced To More In Opposite Direction On [ANSWERED] 20 Two Electrons Are Forced To More In Opposite Direction On](https://media.kunduz.com/media/sug-question-candidate/20211107122233300990-2589424.jpg?h=512)
[ANSWERED] 20 Two Electrons Are Forced To More In Opposite Direction On
Spin and Stability
3. The Balancing Act of Electron Spins
Imagine trying to balance a spinning top. It's much easier if you have another top spinning in the opposite direction to counteract its wobble. Similarly, the pairing of electrons with opposite spins contributes significantly to the stability of atoms. These paired spins create a balanced magnetic field, minimizing the overall energy of the atom and making it less reactive.
When electrons are paired, their magnetic fields cancel each other out, resulting in a net magnetic moment of zero. This cancellation is important for the chemical properties of elements. Elements with unpaired electrons are generally more reactive because these unpaired electrons are eager to form bonds and achieve a more stable, paired configuration.
Consider the noble gases, like helium or neon. They have completely filled electron shells, meaning all their electrons are paired. This stable electron configuration makes them incredibly unreactive — hence the name "noble." They're content with their balanced spins and aren't interested in forming bonds with other elements. On the other hand, elements like sodium or chlorine have unpaired electrons and are highly reactive, always looking to gain or lose an electron to achieve a more stable, paired configuration.
So, next time you see a chemical reaction, remember that it's all about electrons trying to achieve that perfect spin balance. It's a quantum quest for stability, driven by the fundamental principle that opposites attract, at least when it comes to electron spin.

Chemistry Analysis, Reactions, Compounds Britannica
Beyond Atoms
4. From Magnets to Quantum Computing
The concept of electron spin isn't just confined to the realm of individual atoms. It plays a crucial role in the properties of materials and is even paving the way for revolutionary technologies. One of the most obvious examples is magnetism. In ferromagnetic materials, like iron, the spins of many electrons align in the same direction, creating a strong magnetic field. This alignment is what allows us to make permanent magnets and store data on hard drives.
Think about it — without electron spin, we wouldn't have magnets, compasses, or the ability to record information on magnetic media. All of these technologies rely on the collective alignment of electron spins in certain materials. The strength and direction of the magnetic field can be manipulated to store and retrieve information, making our modern digital world possible.
But spin's influence doesn't stop there. Scientists are also exploring the use of electron spin in a new field called spintronics. Spintronics aims to use the spin of electrons, in addition to their charge, to create new electronic devices. This could lead to faster, more energy-efficient computers and other electronic gadgets. Imagine devices that can process information using the spin of electrons, rather than just their charge — that's the promise of spintronics.
And if that wasn't exciting enough, researchers are also investigating the potential of using electron spin in quantum computing. Quantum computers use quantum bits, or qubits, to store and process information. Electron spin is a promising candidate for creating qubits, as it's a stable and well-defined quantum property. Building a quantum computer is a massive technological challenge, but the potential rewards are enormous. It could revolutionize fields like medicine, materials science, and artificial intelligence. So, from magnets to quantum computers, electron spin is a fundamental property that continues to shape our world and drive innovation.
Orbital Diagrams Chemistry Steps
FAQ
5. Your Burning Questions Answered
Q: Does an electron really spin?A: Not in the way we typically think of spinning. It's more of an intrinsic property that gives it angular momentum and a magnetic moment. The term "spin" is just a convenient way to describe this quantum characteristic.
Q: What if there are more than two electrons in an orbital?A: That's not possible! The Pauli Exclusion Principle dictates that each orbital can hold a maximum of two electrons, and they must have opposite spins.
Q: Why is the Pauli Exclusion Principle so important?A: It's fundamental to the structure of matter. Without it, atoms would collapse, chemistry would be different, and life as we know it wouldn't exist!
Q: Is it correct if "Can only two electrons that spin in the opposite direction exist" is used as my keyword term?A: Yes, it directly relates to the core concept being discussed. It's a well-defined scientific concept that's easy to understand for search engines.
